Abstract
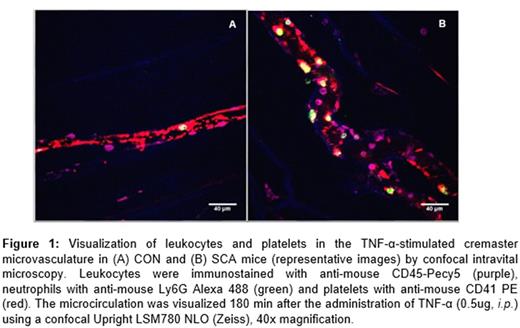
Sickle cell anemia (SCA) pathophysiology results in chronic vascular hemolysis, and an exacerbated inflammatory state. Inflammatory molecule release and pancellular activation in the blood vessel triggers the recruitment of leukocytes and red blood cells (RBC) to the endothelial wall, and the subsequent vaso-occlusive processes that characterize the disease. A potential role for platelets in the initiation of vaso-occlusion is becoming increasingly clear, as these cells are known to form heterocellular aggregates with neutrophils and RBC in the SCA circulation and were recently shown to promote LPS-induced pulmonary vaso-occlusion in SCA mice (Bennewitz et al., JCI Insight 2017). TNF-α-triggered inflammatory processes in the cremaster microcirculation of SCA mice are often used to model vaso-occlusive processes in SCA (Turhan et al., PNAS 2002). As such, we sought to characterize the role of platelets in this model utilizing confocal and conventional intravital microscopy. Control (CON) and SCA male chimeric mice were generated by bone marrow transplantation (from C57BL6 or Berkeley SCA mice) to irradiated C57BL6 mice. After confirmation of their phenotypes, leukocyte recruitment was stimulated, or not, in mice by with the administration of TNF-α (0.5μg, i.p .) or vehicle (saline), and the cremaster microcirculation was observed by confocal and conventional intravital microscopy (the latter was employed for leukocyte adhesion quantification). For confocal microscopy, mice were administered i.v. with anti-CD41 PE, anti-CD45 PeCy-5 and anti-Ly6Gr Alexa 488, for the visualization of platelets, total leukocytes and neutrophils respectively, prior to microscopy. The effect of platelet clearance was investigated using an anti-GPIb-α (anti-CD42b) antibody (Emfret Analytics). Confocal intravital microscopy showed that, while platelets circulate freely in the cremaster microcirculation of unstimulated CON mice, the administration of TNF-α elicits, within 3h, significant platelet recruitment to the endothelium (Fig.1A), in association with the adhesion of some leukocytes (composed of both neutrophils and other leukocytes; 9.1 ± 0.8 leuk adhered 100µm-1). At 3 h post-TNF-α administration to SCA mice (Fig. 1B), considerable quantities of adhered platelets can be seen lining the endothelium and these platelets mediate the adhesion of numerous leukocytes (22.0 ± 1.8 leuk adhered 100µm-1; P<0.01, compared to CON). Of these leukocytes, 60.0±8.2% were neutrophils, and each adhered neutrophil was clearly observed as adhered in association with platelet agglomerations on the venule wall. In contrast, the rolling and adhesion of non-neutrophil leukocytes to the endothelium was rarely associated with the presence of platelet aggregates. To determine whether this platelet recruitment to the endothelium was essential for leukocyte recruitment and subsequent vaso-occlusive processes, SCA mice (n=3, for each group) received TNF-α, together with anti-CD42b (GPIbα) antibody (2μg/g, i.v.) or non-specific IgG, and leukocyte recruitment in the cremaster microcirculation was determined at 1h. Anti-CD42b almost completely depleted platelet numbers in SCA mice within 1 h (data not shown), and this clearance was associated with an inhibition of the adhesion of leukocytes to the venule walls in response to TNF-α when compared to IgG (3.8 ± 0.6 and 16.0 ± 1.6, leuk adhered 100µm-1, respectively, P<0.001). However, unexpectedly, anti-CD42b decreased the survival of SCA mice, but not CON mice, post-TNF-α administration (survival was <3h), possibly due to platelet sequestration in organs, or even due to the inhibition of platelet recruitment to the damaged endothelium. Thus, we present data to suggest that platelet recruitment to the endothelium mediates the secondary adhesion of neutrophils (but not non-neutrophil leukocytes) in the cremaster microcirculation of TNF-α-stimulated SCA mice, a frequently employed model of inflammatory vaso-occlusive processes. The role of the platelets in SCA vascular inflammation is becoming increasingly clear and these cells represent a primary pharmacological target for the prevention of vaso-occlusion in this disease.
Conran: Bayer AG: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal